Question Jon Draw The Major Organic Product
Onlines
Mar 10, 2025 · 6 min read
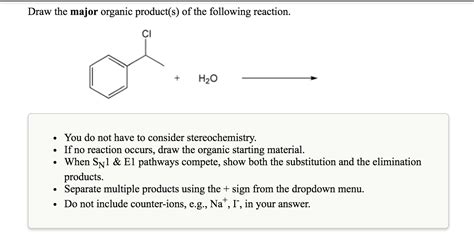
Table of Contents
Question: Jon Draws the Major Organic Product – A Deep Dive into Organic Reaction Mechanisms
Predicting the major organic product of a reaction is a cornerstone of organic chemistry. This skill requires a thorough understanding of reaction mechanisms, including nucleophiles, electrophiles, reaction intermediates, and the influence of steric and electronic effects. This article delves into various reaction types, highlighting strategies to accurately predict the major product when presented with a reaction scheme. We'll explore examples and explain the reasoning behind the formation of the major product, empowering you to confidently tackle similar problems.
Understanding Reaction Mechanisms: The Foundation of Prediction
Before tackling specific reactions, it's crucial to understand the fundamental principles governing organic reactions. Reactions occur through a series of steps involving the breaking and formation of bonds. Identifying the key steps is paramount to predicting the outcome. Let's briefly review some essential concepts:
1. Nucleophiles and Electrophiles: The Dance of Electron Pairs
- Nucleophiles (Nu⁻): Species with a high electron density (lone pairs or π bonds) that donate electrons to electrophiles. They are often negatively charged or possess a partially negative charge. Examples include hydroxide ions (OH⁻), alkoxide ions (RO⁻), and amines (R₃N).
- Electrophiles (E⁺): Species that are electron deficient and accept electrons from nucleophiles. They are often positively charged or possess a partially positive charge. Examples include carbocations (R₃C⁺), carbonyl carbons (C=O), and alkyl halides (R-X).
2. Reaction Intermediates: Transient Species
Many reactions proceed through transient species, such as carbocations, carbanions, and radicals, which are highly reactive and quickly transform into more stable molecules. Understanding the stability of these intermediates is crucial in predicting the major product. Carbocation stability, for instance, follows the order: tertiary > secondary > primary > methyl.
3. Steric Hindrance: Bulky Groups Matter
The spatial arrangement of atoms and groups significantly impacts reaction rates and product formation. Bulky substituents can hinder the approach of reactants, affecting the reaction pathway and leading to a preference for less hindered products. This is particularly important in SN1 and SN2 reactions.
4. Electronic Effects: Resonance and Inductive Effects
Electronic effects, including resonance and inductive effects, influence the reactivity and stability of molecules. Resonance stabilization delocalizes electron density, increasing stability, while inductive effects describe the polarization of electron density through sigma bonds.
Common Reaction Types and Predicting Major Products
Let's examine some common reaction types and strategies for predicting the major organic products:
1. SN1 (Substitution Nucleophilic Unimolecular) Reactions
SN1 reactions proceed through a two-step mechanism involving the formation of a carbocation intermediate. The rate-determining step is the ionization of the alkyl halide to form the carbocation.
- Mechanism: R-X → R⁺ + X⁻ (slow) followed by R⁺ + Nu⁻ → R-Nu (fast)
- Major Product Prediction: The major product is determined by the stability of the carbocation intermediate. More substituted carbocations (tertiary > secondary > primary) are more stable and thus form faster. Therefore, the major product will be the one arising from the most stable carbocation. Rearrangements can also occur to form a more stable carbocation.
Example: The SN1 reaction of tert-butyl bromide with methanol will predominantly yield tert-butyl methyl ether because the tertiary carbocation is highly stable.
2. SN2 (Substitution Nucleophilic Bimolecular) Reactions
SN2 reactions are concerted, meaning they occur in a single step. The nucleophile attacks the substrate from the backside, leading to inversion of configuration.
- Mechanism: Nu⁻ + R-X → [Nu···R···X]‡ → Nu-R + X⁻
- Major Product Prediction: Steric hindrance plays a significant role. Less hindered substrates react faster. Primary alkyl halides undergo SN2 reactions readily, while tertiary alkyl halides are hindered and react very slowly or not at all. The product will have inverted stereochemistry compared to the reactant.
Example: The SN2 reaction of methyl bromide with sodium hydroxide will yield methanol with complete inversion of configuration if the methyl bromide was chiral.
3. E1 (Elimination Unimolecular) Reactions
E1 reactions also proceed through a carbocation intermediate, leading to the formation of an alkene.
- Mechanism: R-X → R⁺ + X⁻ (slow) followed by R⁺ → Alkene + H⁺ (fast)
- Major Product Prediction: The major product will be the most substituted alkene (Zaitsev's rule). The more substituted alkene is more stable due to hyperconjugation.
Example: The E1 reaction of 2-bromo-2-methylpropane will predominantly yield 2-methylpropene (isobutylene), the more substituted alkene.
4. E2 (Elimination Bimolecular) Reactions
E2 reactions are concerted, with the base abstracting a proton and the leaving group departing simultaneously.
- Mechanism: Base + R-X → Alkene + Base-H + X⁻
- Major Product Prediction: The major product is usually the most substituted alkene (Zaitsev's rule), but steric factors can influence the outcome. Anti-periplanar geometry is preferred, meaning the proton and leaving group must be on opposite sides of the molecule.
Example: The E2 reaction of 2-bromobutane with a strong base like potassium tert-butoxide will predominantly yield 2-butene (the more substituted alkene).
5. Electrophilic Aromatic Substitution Reactions
These reactions involve the substitution of a hydrogen atom on an aromatic ring with an electrophile.
- Mechanism: Electrophilic attack, followed by proton loss.
- Major Product Prediction: The major product is determined by the directing effects of substituents already present on the ring. Activating groups (e.g., -OH, -NH₂) direct electrophiles to the ortho and para positions, while deactivating groups (e.g., -NO₂, -COOH) direct electrophiles to the meta position.
Example: Nitration of toluene will predominantly yield ortho and para-nitrotoluene because the methyl group is an activating group.
6. Addition Reactions to Alkenes and Alkynes
Addition reactions involve the breaking of a π bond and the formation of two new σ bonds.
- Mechanism: Electrophilic attack on the π bond, followed by nucleophilic attack.
- Major Product Prediction: Markovnikov's rule applies to the addition of unsymmetrical reagents to alkenes. The electrophile adds to the carbon atom with the greater number of hydrogen atoms. Steric hindrance can also affect regioselectivity.
Example: Addition of HBr to propene will predominantly yield 2-bromopropane (Markovnikov's product).
Advanced Considerations: Beyond the Basics
Several other factors can influence the major product formation:
- Solvent Effects: The solvent can significantly impact reaction rates and selectivity. Polar protic solvents favor SN1 and E1 reactions, while polar aprotic solvents favor SN2 reactions.
- Temperature: Higher temperatures often favor elimination reactions over substitution reactions.
- Base Strength: Strong bases favor elimination reactions, while weak bases favor substitution reactions.
- Leaving Group Ability: Good leaving groups (e.g., I⁻, Br⁻, Cl⁻, TsO⁻) facilitate both substitution and elimination reactions.
Practical Tips for Predicting Major Organic Products
- Identify the functional groups: Recognizing the functional groups present in the reactants is the first step.
- Determine the type of reaction: Is it a substitution, elimination, addition, or another type of reaction?
- Identify the nucleophile and electrophile (if applicable): This helps to determine the reaction mechanism.
- Consider the stability of intermediates: Carbocations, carbanions, and other intermediates can significantly impact the outcome.
- Account for steric and electronic effects: These effects influence both reactivity and selectivity.
- Apply relevant rules: Markovnikov's rule, Zaitsev's rule, and other rules can help predict the major product.
- Draw the mechanism: Writing out the step-by-step mechanism can help you visualize the reaction pathway and identify the major product.
- Practice, practice, practice: The more examples you work through, the better you'll become at predicting the major organic product.
By carefully considering these factors and practicing diligently, you will significantly improve your ability to accurately predict the major organic product in a wide variety of reactions. Remember, predicting the major product is a skill that develops with experience and a thorough understanding of reaction mechanisms. Consistent practice and a systematic approach are key to mastery.
Latest Posts
Latest Posts
-
The Great Gatsby Chapter By Chapter Summary
Mar 10, 2025
-
1 3 Practice Measuring Segments Form G
Mar 10, 2025
-
Cold War Events And Policies Worksheet Answers
Mar 10, 2025
-
Ap Statistics Transformations To Achieve Linearity Worksheet
Mar 10, 2025
-
Match The Link State To The Interface And Protocol Status
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Question Jon Draw The Major Organic Product . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
