Select The Correct Iupac Name For The Cycloalkane
Onlines
Mar 19, 2025 · 5 min read
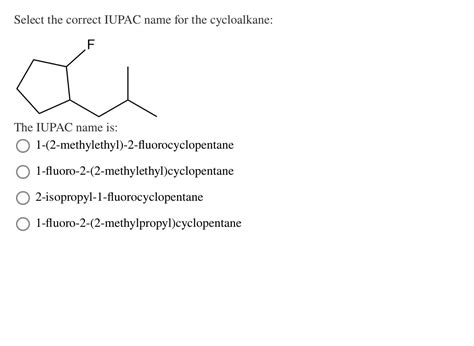
Table of Contents
Selecting the Correct IUPAC Name for a Cycloalkane: A Comprehensive Guide
Understanding the nomenclature of cycloalkanes is crucial for anyone working in organic chemistry. The International Union of Pure and Applied Chemistry (IUPAC) provides a systematic approach to naming these cyclic hydrocarbons, ensuring unambiguous communication within the scientific community. This comprehensive guide will delve into the intricacies of IUPAC nomenclature for cycloalkanes, equipping you with the skills to correctly name even the most complex structures.
Understanding the Basics: Cycloalkanes and their Structure
Cycloalkanes are saturated hydrocarbons containing a closed ring of carbon atoms. Unlike their linear alkane counterparts, they possess a cyclic structure, significantly impacting their properties and nomenclature. The simplest cycloalkane is cyclopropane (C₃H₆), followed by cyclobutane (C₄H₈), cyclopentane (C₅H₁₀), and so on. The general formula for a cycloalkane is CₙH₂ₙ, where 'n' represents the number of carbon atoms in the ring.
Key Features Affecting IUPAC Naming:
Several key features influence the IUPAC name of a cycloalkane:
- Ring Size: The number of carbon atoms in the ring dictates the parent name (e.g., cyclopropane, cyclobutane).
- Substituents: Any atoms or groups attached to the ring are considered substituents and must be named and located precisely.
- Multiple Substituents: If more than one substituent is present, their positions and alphabetical order must be carefully considered.
- Complex Substituents: Substituents themselves can be complex molecules, requiring their own systematic naming.
- Stereochemistry: The spatial arrangement of substituents (cis/trans or R/S configurations) may also be incorporated into the name, adding further layers of complexity.
Step-by-Step Guide to IUPAC Naming of Cycloalkanes
Let's break down the process of assigning the correct IUPAC name to a cycloalkane, covering various scenarios and complexities.
Step 1: Identifying the Parent Cycloalkane:
This is the foundational step. Count the number of carbon atoms in the ring. The parent name is simply "cyclo" followed by the alkane name corresponding to the number of carbons (e.g., cyclohexane for a six-membered ring).
Step 2: Identifying and Numbering Substituents:
Locate all substituents attached to the cycloalkane ring. Number the carbon atoms of the ring starting at the point of attachment of the first substituent, proceeding in the direction that leads to the lowest possible numbers for the other substituents. If there are multiple substituents, the numbering should be such that they receive the lowest set of locants.
Step 3: Naming Substituents:
Name each substituent according to its IUPAC name. This might involve simple alkyl groups (methyl, ethyl, propyl, etc.) or more complex functional groups.
Step 4: Alphabetical Ordering of Substituents:
Arrange the substituents alphabetically, ignoring prefixes like di-, tri-, tetra-, etc., except for iso-, sec-, and tert-. The prefixes are considered only for alphabetization after the basic alkyl group name.
Step 5: Combining the Name:
Combine all the components to create the complete IUPAC name. The format generally follows this order: number-substituent-number-substituent-…-cycloalkane. Numbers are separated by hyphens, and substituents are separated by commas.
Step 6: Addressing Stereochemistry (Cis/Trans Isomerism):
For cycloalkanes with two or more substituents, stereochemistry becomes relevant. If the substituents are on the same side of the ring, they are designated as cis. If they are on opposite sides, they are designated as trans. This stereochemical descriptor is added as a prefix to the name (e.g., cis-1,2-dimethylcyclohexane).
Step 7: Advanced Scenarios - Complex Substituents:
When complex substituents are present, these must be named individually following the same IUPAC rules before being incorporated into the cycloalkane name.
Examples: Illustrating the Naming Process
Let's work through several examples to solidify our understanding.
Example 1: Simple Substitution
Consider a cyclohexane ring with a single methyl group attached to one of the carbon atoms.
- Parent: Cyclohexane
- Substituent: Methyl
- Numbering: The methyl group is at position 1 (it doesn't matter which carbon you start numbering from in this case, as all positions are equivalent).
- Name: Methylcyclohexane
Example 2: Multiple Substituents
Imagine a cyclopentane ring with a methyl group on carbon 1 and an ethyl group on carbon 3.
- Parent: Cyclopentane
- Substituents: Methyl, ethyl
- Numbering: The numbering starts at the methyl group to get the lowest possible locants.
- Alphabetical Order: Ethyl precedes methyl alphabetically.
- Name: 1-Ethyl-3-methylcyclopentane
Example 3: Cis/Trans Isomerism
Consider 1,2-dimethylcyclohexane. This can exist as two isomers: cis and trans.
- cis-1,2-dimethylcyclohexane: Both methyl groups are on the same side of the ring plane.
- trans-1,2-dimethylcyclohexane: The methyl groups are on opposite sides of the ring plane.
Example 4: Complex Substituents
Consider a cyclohexane ring with a 2-methylpropyl group attached.
- Parent: Cyclohexane
- Substituent: 2-Methylpropyl (isobutyl)
- Numbering: Start numbering from the carbon atom where the isobutyl group is attached.
- Name: 1-(2-Methylpropyl)cyclohexane or 1-Isobutylcyclohexane
Tackling Challenging Scenarios
Here are some more complex scenarios encountered in cycloalkane naming:
- Rings within Rings (Spiro Compounds): These require specific naming conventions involving "spiro" prefixes and careful numbering.
- Fused Ring Systems: Cycloalkanes can be fused together to form more complex structures, necessitating different nomenclature rules.
- Bridged Ring Systems: These involve carbon atoms shared between multiple rings. This requires the use of specific nomenclature systems like bicyclic or polycyclic nomenclature.
- Heterocyclic Rings: If the ring contains atoms other than carbon (e.g., oxygen, nitrogen, sulfur), the naming conventions become more elaborate, incorporating prefixes to reflect the heteroatoms.
Advanced Techniques and Resources
Mastering IUPAC nomenclature requires practice and familiarity with the rules. Several resources can help refine your skills:
- IUPAC Nomenclature Books: These provide detailed guidelines and examples for various chemical structures.
- Online Chemical Databases: Databases like PubChem or ChemSpider allow you to search for compounds and view their IUPAC names, providing excellent practice.
- Organic Chemistry Textbooks: Most organic chemistry textbooks dedicate significant chapters to nomenclature, offering numerous practice problems.
Conclusion
The correct IUPAC naming of cycloalkanes is a fundamental skill in organic chemistry. By following the systematic approach outlined in this guide, you can confidently assign accurate and unambiguous names to even the most complex cycloalkane structures. Remember, consistent practice and familiarity with the rules are key to mastering this essential aspect of organic chemistry nomenclature. The ability to accurately name cycloalkanes is not only essential for clear communication in the scientific community but also vital for understanding and predicting the properties and reactivity of these important organic molecules. Continuous learning and the utilization of available resources are vital for consistent improvement and mastery of this crucial aspect of organic chemistry.
Latest Posts
Latest Posts
-
You Have Just Installed An Anti Malware Program On Your Pc
Mar 19, 2025
-
Crime Scene To Courtroom Review Puzzle Template
Mar 19, 2025
-
Correctly Label The Parts Of An Exocrine Gland
Mar 19, 2025
-
Which Statement Is True About Presidential Decision Making
Mar 19, 2025
-
What Is The Main Point Of The Quizmaster Study
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Select The Correct Iupac Name For The Cycloalkane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
