Which Of The Following Represents The Enantiomer Of A
Onlines
Apr 06, 2025 · 5 min read
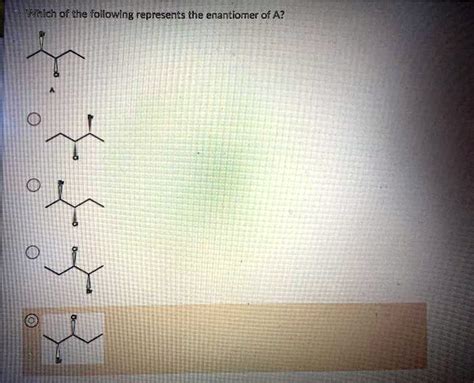
Table of Contents
Which of the Following Represents the Enantiomer of A? A Comprehensive Guide to Chirality and Stereochemistry
Understanding enantiomers is crucial in organic chemistry and related fields like biochemistry and pharmacology. Enantiomers, also known as optical isomers, are pairs of molecules that are non-superimposable mirror images of each other. This seemingly subtle difference can have profound impacts on the properties and behavior of these molecules, particularly in biological systems. This article will delve into the concept of enantiomers, exploring how to identify them and highlighting their significance.
What are Enantiomers?
Before diving into identifying enantiomers, let's solidify our understanding of the fundamental concepts. Molecules exhibiting chirality possess a property called handedness. Think of your hands – they are mirror images, but you cannot superimpose one onto the other. Similarly, chiral molecules have a non-superimposable mirror image. This chirality arises due to the presence of stereocenters (also called chiral centers). A stereocenter is typically a carbon atom bonded to four different groups.
The presence of a single stereocenter guarantees the existence of a pair of enantiomers. Multiple stereocenters lead to a more complex situation with the possibility of diastereomers (stereoisomers that are not mirror images) in addition to enantiomers.
Identifying Enantiomers: A Step-by-Step Approach
Let's outline a systematic method for identifying enantiomers among a given set of molecules:
-
Identify Chiral Centers: The first step involves carefully examining each molecule in the set to locate any carbon atoms bonded to four different groups. These are your chiral centers. Remember that identical groups on a carbon atom negate chirality at that center.
-
Draw Mirror Images: For each molecule containing a chiral center, draw its mirror image. This is crucial for visually comparing whether it's an enantiomer or not. It's often helpful to use wedges and dashes to represent stereochemistry clearly (wedges for groups projecting out of the plane and dashes for groups projecting behind the plane).
-
Assess Superimposability: Attempt to superimpose the original molecule and its mirror image. If they are non-superimposable – meaning you cannot perfectly overlap all atoms and bonds – you have a pair of enantiomers. If they are superimposable, they are identical and not enantiomers.
-
Consider Multiple Chiral Centers: When dealing with molecules possessing multiple chiral centers, the process becomes more nuanced. You must carefully compare the configuration at each chiral center. If all configurations are reversed in the mirror image, you have a pair of enantiomers. If some are reversed and others are not, you have diastereomers.
Properties of Enantiomers
Enantiomers share many physical properties like melting point, boiling point, and solubility in achiral solvents. However, they differ significantly in their interactions with plane-polarized light and in their interactions with other chiral molecules.
-
Optical Activity: This is the key distinguishing property. Enantiomers rotate plane-polarized light in opposite directions. One enantiomer rotates the light clockwise (+ or dextrorotatory), and the other rotates it counterclockwise (− or levorotatory). The extent of rotation is quantified by the specific rotation, denoted as [α].
-
Chiral Recognition: Enantiomers interact differently with other chiral molecules. This is particularly relevant in biological systems. Enzymes, receptors, and other biological molecules are chiral, and they often exhibit selective binding or reactivity with one enantiomer over the other. This explains why a specific enantiomer of a drug might be highly effective while its mirror image is ineffective or even toxic.
Practical Examples and Applications
Let's illustrate these concepts with some examples. Suppose we have a molecule "A" with a specific stereochemical configuration. We need to identify which of the other molecules presented are its enantiomer. We would carefully follow the steps outlined above:
(Example 1: Simple case with one chiral center)
Let's assume molecule A has a chiral carbon bonded to –CH3, -OH, -COOH, and -H groups in a specific arrangement. To find its enantiomer, we'd draw the mirror image and check for non-superimposability. Only the mirror image with precisely reversed configuration at the chiral carbon would be its enantiomer.
(Example 2: More complex case with multiple chiral centers)
Consider molecule A with two chiral centers. It might have several stereoisomers: one enantiomer and two diastereomers. Identifying the enantiomer requires a careful analysis of the stereochemistry at each chiral center. Switching the configuration at both chiral centers will give you the enantiomer. Switching the configuration at just one center would produce a diastereomer.
Applications in different fields:
-
Pharmacology: The different biological activities of enantiomers have profound implications in drug design and development. Often, only one enantiomer of a drug is therapeutically active, while the other may be inactive or even harmful. This has led to the development of single-enantiomer drugs, which offer improved efficacy and reduced side effects. Thalidomide is a notorious example of the different effects of enantiomers, where one was effective as a sedative, and the other caused severe birth defects.
-
Biochemistry: Many biologically important molecules are chiral, including amino acids, sugars, and nucleic acids. The specific stereochemistry of these molecules is essential for their biological function. For instance, enzymes often exhibit stereospecificity, catalyzing reactions with only one enantiomer of a substrate.
-
Food Science: Chiral molecules influence the taste and smell of food. Enantiomers can have significantly different sensory properties, leading to different flavour profiles.
-
Materials Science: Chirality plays an important role in the properties of materials. For example, chiral crystals can exhibit unique optical and electronic properties.
Advanced Concepts and Considerations
-
Racemic Mixtures: A mixture containing equal amounts of both enantiomers is called a racemic mixture. Racemic mixtures are optically inactive because the rotations of the enantiomers cancel each other out.
-
Resolution: This is the process of separating a racemic mixture into its individual enantiomers. Several techniques exist for resolution, including chiral chromatography and crystallization using chiral resolving agents.
-
Absolute Configuration: This refers to the three-dimensional arrangement of atoms in a chiral molecule. It is typically denoted using the Cahn-Ingold-Prelog (CIP) system (R/S nomenclature).
Conclusion
Identifying enantiomers requires a thorough understanding of chirality, stereocenters, and the concept of non-superimposable mirror images. While seemingly subtle, the difference between enantiomers can lead to profound differences in their properties and applications, particularly within biological contexts. The systematic approach outlined in this article provides a solid foundation for confidently identifying enantiomers and appreciating their significance in various scientific disciplines. Further exploration of stereochemistry and advanced concepts like diastereomers and meso compounds will enrich your understanding even further. This knowledge is crucial for anyone working in fields involving molecules with chiral centers, emphasizing the importance of careful stereochemical analysis and consideration in all related applications.
Latest Posts
Latest Posts
-
Experiment 5 The Importance Of Cell Cycle Control
Apr 07, 2025
-
2 08 Quiz Rhetoric Develops Purpose And Viewpoint
Apr 07, 2025
-
2 5b Exponential Function Context And Data Modeling
Apr 07, 2025
-
Amoeba Sisters Video Recap Introduction To Cells Answer Key
Apr 07, 2025
-
Which Of The Following Strategies Would Effectively Reduce Racism
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Represents The Enantiomer Of A . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.