Which Reaction Sequence Best Accomplishes This Transformation
Onlines
Mar 17, 2025 · 6 min read
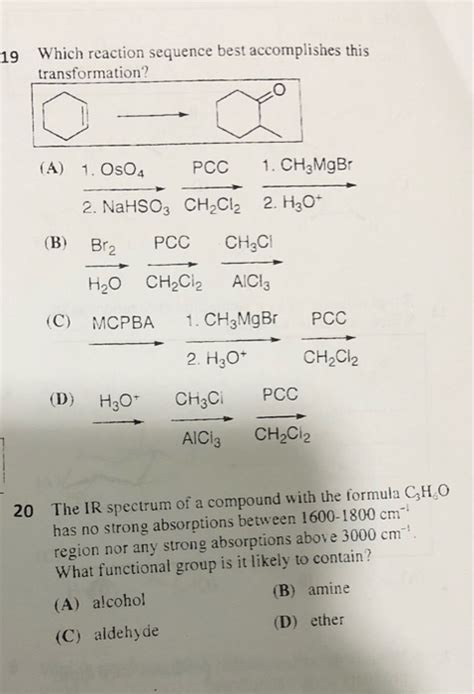
Table of Contents
Which Reaction Sequence Best Accomplishes This Transformation? A Comprehensive Guide
Choosing the optimal reaction sequence for a specific organic transformation is a crucial skill in organic chemistry. The best sequence minimizes steps, maximizes yield, and utilizes reactions with high selectivity and predictability. This article delves into the strategic thinking behind selecting the most efficient reaction pathway, focusing on various factors that influence the decision-making process. We will examine several common transformations and analyze different approaches, highlighting the advantages and disadvantages of each strategy.
Understanding the Target Molecule and Starting Material
Before even considering potential reaction sequences, a thorough understanding of both the starting material and the target molecule is paramount. This involves:
-
Identifying Functional Groups: Precisely identify all functional groups present in both the starting material and the desired product. This is fundamental for choosing reactions that can introduce, modify, or remove specific functional groups.
-
Analyzing the Carbon Skeleton: Determine if the carbon skeleton needs significant modification, such as the formation or breaking of carbon-carbon bonds. This will greatly influence the choice of reaction sequences. A simple modification might only require a few steps, while a complex restructuring might require a multi-step approach involving several intermediate products.
-
Stereochemistry Considerations: If stereochemistry is crucial for the target molecule (e.g., specific enantiomers or diastereomers are required), the selected reactions must ensure stereoselectivity. This often necessitates the use of chiral catalysts or reagents.
-
Protecting Groups: Often, functional groups that are reactive under certain conditions need to be temporarily protected to prevent unwanted side reactions. Carefully consider which protecting groups are appropriate and how to introduce and remove them efficiently.
Common Reaction Strategies and Their Applications
Let's examine some common reaction strategies used in organic synthesis and how they can be employed to accomplish specific transformations.
1. Functional Group Interconversion (FGI):
FGI focuses on transforming one functional group into another. This often involves a series of oxidation, reduction, or nucleophilic/electrophilic substitution reactions.
Example: Converting an alcohol to a halide. This could be accomplished through several routes:
- Reaction with Thionyl Chloride (SOCl2): A direct conversion, often producing the corresponding alkyl chloride.
- Reaction with Phosphorus tribromide (PBr3): Similarly, this leads to the alkyl bromide.
- Reaction with Hydrogen Halide (HCl, HBr): This route is often less efficient and can lead to side reactions.
The choice depends on the specific alcohol, desired halide, and the presence of other reactive groups in the molecule. SOCl2 and PBr3 are generally preferred for their efficiency and selectivity.
2. Carbon-Carbon Bond Formation:
Constructing the carbon skeleton is a cornerstone of organic synthesis. Numerous reactions facilitate this, including:
-
Grignard Reactions: Forming new carbon-carbon bonds by reacting a Grignard reagent (organomagnesium halide) with a carbonyl compound (aldehyde, ketone, ester). Highly versatile for building various carbon skeletons.
-
Wittig Reaction: Converting aldehydes and ketones to alkenes using a phosphorous ylide. Excellent for creating specific alkene geometries (E or Z).
-
Aldol Condensation: Forming carbon-carbon bonds between two carbonyl compounds through enolate formation and nucleophilic addition. Produces β-hydroxy carbonyl compounds, which can further be dehydrated to α,β-unsaturated carbonyl compounds.
-
Diels-Alder Reaction: A [4+2] cycloaddition reaction between a diene and a dienophile, forming a six-membered ring. Highly stereoselective and useful for creating complex cyclic systems.
The choice of reaction depends heavily on the desired structure and functionality. For example, if a specific alkene geometry is needed, the Wittig reaction might be the preferred choice.
3. Ring-Opening and Ring-Closing Reactions:
These reactions are vital for manipulating cyclic structures.
-
Ring-Opening Metathesis Polymerization (ROMP): A powerful technique for creating polymers from cyclic alkenes.
-
Ring-Closing Metathesis (RCM): The reverse of ROMP, forming cyclic alkenes from acyclic dienes. Often used in the synthesis of complex natural products.
-
Epoxide Ring Opening: Nucleophilic attack on an epoxide ring can open the ring, creating a new carbon-oxygen bond. The regiochemistry and stereochemistry of the reaction are influenced by the nucleophile and the epoxide's substituents.
4. Oxidation and Reduction Reactions:
These reactions modify the oxidation state of functional groups.
-
Oxidations: Converting alcohols to aldehydes or ketones (e.g., using PCC, Jones reagent, or Swern oxidation), aldehydes to carboxylic acids (e.g., using KMnO4 or CrO3), and alkenes to epoxides (e.g., using mCPBA).
-
Reductions: Converting ketones and aldehydes to alcohols (e.g., using NaBH4 or LiAlH4), alkenes to alkanes (e.g., using catalytic hydrogenation), and nitro groups to amines (e.g., using catalytic reduction).
Strategic Considerations for Reaction Sequence Design
Designing an efficient reaction sequence demands strategic thinking:
-
Retrosynthetic Analysis: Working backward from the target molecule to identify key intermediates and potential precursors is a powerful approach. This allows for a systematic breakdown of the synthesis into manageable steps.
-
Protecting Groups: When multiple functional groups are present, protecting groups might be essential to prevent unwanted reactions. The choice of protecting group should be based on compatibility with the other reactions in the sequence and ease of removal.
-
Yield Optimization: Each step in a reaction sequence contributes to the overall yield. Prioritizing reactions with high yields is crucial for maximizing the overall efficiency of the synthesis.
-
Reagent Costs and Availability: The cost and availability of reagents are practical considerations. Choosing readily available and cost-effective reagents is often preferable.
-
Reaction Conditions: The reaction conditions (temperature, solvent, pH) must be carefully considered. Incompatible conditions might lead to undesired side reactions or low yields.
-
Purification Strategies: Planning how to purify intermediate products is crucial. Choosing purification methods that are efficient and compatible with the scale of the synthesis is vital.
Case Studies: Illustrative Examples
Let's consider a few specific transformations to illustrate the practical application of these principles.
Transformation 1: Converting a simple alcohol to a carboxylic acid:
Multiple pathways exist:
-
Path A: Oxidation (direct): Using a strong oxidizing agent like Jones reagent (CrO3/H2SO4) can directly convert the alcohol to the carboxylic acid. However, this method can be harsh and may lead to side reactions.
-
Path B: Oxidation to aldehyde, then to carboxylic acid: A milder approach involves initially oxidizing the alcohol to an aldehyde (e.g., using PCC) followed by further oxidation of the aldehyde to the carboxylic acid (e.g., using Ag2O). This offers better control and selectivity.
Transformation 2: Synthesizing a specific substituted cyclohexane:
This might require a multi-step synthesis involving reactions like Diels-Alder cycloaddition, followed by functional group interconversions. Careful consideration of regioselectivity and stereoselectivity is necessary during the Diels-Alder step. Appropriate protecting group strategies may be required if other functional groups are present.
Transformation 3: Forming a complex natural product:
The synthesis of complex natural products often requires elaborate reaction sequences involving multiple carbon-carbon bond-forming reactions, ring-closing metathesis, stereoselective reductions, and oxidations. Retrosynthetic analysis is crucial for planning such complex syntheses.
Conclusion
Selecting the optimal reaction sequence for a specific transformation is a complex process requiring a deep understanding of organic chemistry principles and strategic thinking. Careful consideration of the starting material, target molecule, available reactions, yield optimization, and practical considerations are all crucial elements. Systematic approaches like retrosynthetic analysis are powerful tools for simplifying the design process. By mastering these strategies, organic chemists can efficiently and effectively synthesize a wide range of molecules. The examples provided highlight the importance of evaluating different options and selecting the best approach based on the specific circumstances of each transformation. The field is constantly evolving with new reactions and methodologies emerging, so continuous learning and adaptation are essential.
Latest Posts
Latest Posts
-
Montse Quiere Que Su Dormitorio Elegante
Mar 18, 2025
-
Code Standards And Practices 3 Lesson 1
Mar 18, 2025
-
Mr Barker Enjoys A Comfortable Retirement Income
Mar 18, 2025
-
Afterlife The Strange Science Of Decay Answer Key
Mar 18, 2025
-
Unit 3 Progress Check Frq Part A Ap Calculus
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Reaction Sequence Best Accomplishes This Transformation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
