Write The Reaction For The Formation Of Fencs2+
Onlines
Apr 07, 2025 · 6 min read
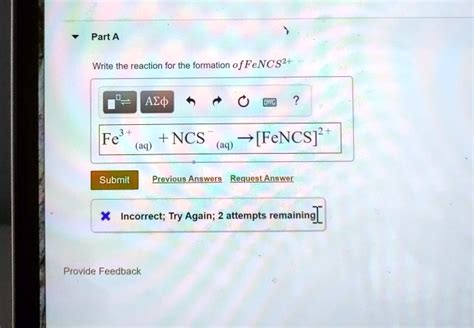
Table of Contents
The Formation of FeNCS²⁺: A Deep Dive into Reaction Mechanisms and Spectrophotometric Analysis
The formation of the iron(III) thiocyanate complex ion, FeNCS²⁺, is a classic example of a coordination complex reaction frequently studied in chemistry education. Its vibrant red color makes it visually appealing, and the reaction's equilibrium constant allows for straightforward spectrophotometric analysis, offering valuable insights into equilibrium principles and chemical kinetics. This article provides a comprehensive overview of the FeNCS²⁺ formation reaction, covering its mechanism, influencing factors, and applications in analytical chemistry.
Understanding the Reaction: Fe³⁺ + SCN⁻ <=> FeNCS²⁺
The reaction's core involves the interaction between ferric ions (Fe³⁺) and thiocyanate ions (SCN⁻) to yield the iron(III) thiocyanate complex ion, FeNCS²⁺. This is a reversible reaction, existing in equilibrium:
Fe³⁺(aq) + SCN⁻(aq) <=> FeNCS²⁺(aq)
The equilibrium lies to the right, indicating that a significant amount of the complex ion forms under suitable conditions. The intensity of the red color directly relates to the concentration of FeNCS²⁺, making it a convenient indicator of the reaction's progress and equilibrium position.
The Nature of the FeNCS²⁺ Complex Ion
FeNCS²⁺ is a coordination complex, where the central iron(III) ion acts as a Lewis acid (electron-pair acceptor), coordinating with the thiocyanate ion, which acts as a Lewis base (electron-pair donor). The bonding involves the donation of a lone pair of electrons from the sulfur atom (or less commonly, the nitrogen atom) of the SCN⁻ ion to an empty orbital in the Fe³⁺ ion. This forms a coordinate covalent bond. The structure of the complex is often depicted as linear, although slight deviations might occur due to steric effects.
Factors Influencing FeNCS²⁺ Formation
Several factors significantly impact the formation and concentration of FeNCS²⁺ at equilibrium:
1. Concentration of Reactants:
According to the law of mass action, increasing the concentration of either Fe³⁺ or SCN⁻ will shift the equilibrium to the right, leading to a higher concentration of FeNCS²⁺ and a more intense red color. Conversely, decreasing the concentration of either reactant shifts the equilibrium to the left, resulting in a less intense color. This direct relationship is fundamental to the spectrophotometric analysis of the reaction.
2. Temperature:
The formation of FeNCS²⁺ is an exothermic reaction. Applying Le Chatelier's principle, increasing the temperature shifts the equilibrium to the left, reducing the concentration of FeNCS²⁺. Conversely, lowering the temperature favors the forward reaction, leading to a higher FeNCS²⁺ concentration. Careful temperature control is therefore crucial for accurate quantitative analysis.
3. pH:
The concentration of free Fe³⁺ ions is significantly influenced by the pH of the solution. Fe³⁺ readily undergoes hydrolysis at higher pH values, forming various hydroxo complexes like Fe(OH)²⁺, Fe(OH)₂⁺, and even precipitated Fe(OH)₃. This reduces the concentration of free Fe³⁺ available to react with SCN⁻, thus decreasing the FeNCS²⁺ formation. Therefore, maintaining a sufficiently low pH (generally acidic conditions) is essential to ensure that a significant amount of Fe³⁺ remains in the solution for the reaction to proceed effectively.
4. Presence of Other Ligands:
The addition of other ligands that can coordinate with Fe³⁺ will compete with SCN⁻ for the metal ion. These ligands, if they have stronger coordinating abilities than SCN⁻, will effectively reduce the concentration of FeNCS²⁺. This competition is a key aspect to consider when designing experiments and interpreting results. For instance, the presence of fluoride, oxalate, or EDTA (ethylenediaminetetraacetic acid) ions would significantly interfere with the formation of FeNCS²⁺.
Spectrophotometric Analysis of FeNCS²⁺
The intense red color of FeNCS²⁺ makes it ideally suited for spectrophotometric analysis, a technique using light absorption to determine the concentration of a colored solution. The Beer-Lambert Law forms the foundation of this analysis:
A = εbc
Where:
- A is the absorbance of the solution
- ε is the molar absorptivity (a constant specific to the FeNCS²⁺ complex at a given wavelength)
- b is the path length of the light through the solution (the width of the cuvette)
- c is the concentration of FeNCS²⁺
By measuring the absorbance of a solution containing FeNCS²⁺ at its maximum absorption wavelength (typically around 450 nm), and knowing the values of ε and b, we can calculate the concentration of the complex ion. This concentration can then be used to determine the equilibrium constant (K) for the reaction.
Determining the Equilibrium Constant (K)
The equilibrium constant (K) for the FeNCS²⁺ formation reaction is defined as:
K = [FeNCS²⁺] / ([Fe³⁺][SCN⁻])
To determine K, we need to know the equilibrium concentrations of Fe³⁺, SCN⁻, and FeNCS²⁺. This can be achieved through several methods:
- Method of Initial Rates: By measuring the initial rate of FeNCS²⁺ formation under varying initial concentrations of Fe³⁺ and SCN⁻, the reaction order can be determined and used to calculate K.
- Spectrophotometric Method: This is the most common method. By measuring the absorbance of solutions with known initial concentrations of Fe³⁺ and SCN⁻, the equilibrium concentration of FeNCS²⁺ can be determined using the Beer-Lambert Law. Using stoichiometry, the equilibrium concentrations of Fe³⁺ and SCN⁻ can then be calculated. Substituting these values into the equilibrium expression yields K.
Applications of FeNCS²⁺ Formation Reaction
The FeNCS²⁺ formation reaction and its spectrophotometric analysis have several applications:
- Teaching Equilibrium Principles: It's a valuable tool for demonstrating concepts of equilibrium, Le Chatelier's principle, and the law of mass action.
- Analytical Chemistry: It serves as a useful method for determining the concentration of either Fe³⁺ or SCN⁻ ions in a sample.
- Kinetic Studies: The reaction's rate can be studied to explore the reaction mechanism and determine the rate constant.
- Exploring Coordination Chemistry: The reaction provides a practical example of coordination complex formation and the influence of various factors on complex stability.
Advanced Considerations and Further Exploration
The seemingly simple FeNCS²⁺ formation reaction actually exhibits complexities not always covered in introductory chemistry. Further investigations can explore:
- Higher-Order Complexes: At higher concentrations of SCN⁻, higher-order complexes such as Fe(NCS)₂⁺ and Fe(NCS)₃ can form, influencing the overall equilibrium.
- Effect of Ionic Strength: The ionic strength of the solution affects the activity coefficients of the ions, consequently altering the equilibrium constant. The Debye-Hückel equation can be used to correct for this effect.
- Solvent Effects: The reaction kinetics and equilibrium constant are sensitive to the solvent used. Using different solvents can reveal further insights into the reaction mechanism.
- Spectroscopic Techniques: Techniques beyond visible spectrophotometry, like UV-Vis spectroscopy, can provide more detailed information about the electronic transitions within the FeNCS²⁺ complex and its structure.
Conclusion
The formation of FeNCS²⁺ is a seemingly simple yet surprisingly rich chemical reaction. Its visual appeal coupled with its straightforward quantitative analysis makes it an invaluable tool in chemical education and analytical chemistry. Understanding the reaction mechanism, influencing factors, and the spectrophotometric techniques used for its analysis provides a strong foundation for further exploration of coordination chemistry, equilibrium principles, and chemical kinetics. The depth of study into this reaction opens doors to advanced concepts and techniques, solidifying its position as a fundamental and versatile example in the chemical sciences.
Latest Posts
Latest Posts
-
How Can A Theme And Variations Form Be Schematically Outlined
Apr 09, 2025
-
Gastric Distention Will Most Likely Occur
Apr 09, 2025
-
Drawing Pictures With Piecewise Functions Answer Key
Apr 09, 2025
-
Stochastic Calculus University Of Nebraska Lincoln
Apr 09, 2025
-
The Characters In The Most Dangerous Game
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Write The Reaction For The Formation Of Fencs2+ . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.