Writing The Rate Law Implied By A Simple Mechanism
Onlines
Mar 26, 2025 · 6 min read
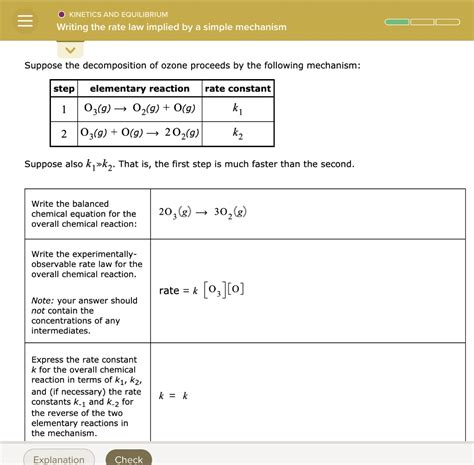
Table of Contents
Writing the Rate Law Implied by a Simple Mechanism
Understanding how to derive the rate law from a proposed reaction mechanism is a cornerstone of chemical kinetics. It bridges the gap between the microscopic world of colliding molecules and the macroscopic world of observable reaction rates. While complex mechanisms can lead to intricate rate laws, mastering the process for simple mechanisms is crucial for tackling more challenging scenarios. This article provides a comprehensive guide to deriving rate laws from simple mechanisms, highlighting key concepts and common pitfalls.
What is a Reaction Mechanism?
A reaction mechanism is a detailed step-by-step description of how a chemical reaction proceeds at the molecular level. It outlines the individual elementary steps, or elementary reactions, that constitute the overall reaction. Each elementary step involves a single collision or unimolecular event and has its own rate law determined directly by its molecularity (the number of molecules involved in the step).
Unlike overall reactions, which might involve multiple steps and complex dependencies, elementary reactions follow straightforward rate laws. For example:
- Unimolecular elementary reaction (A → products): The rate law is directly proportional to the concentration of reactant A: Rate = k[A], where k is the rate constant.
- Bimolecular elementary reaction (A + B → products): The rate law is proportional to the product of the concentrations of reactants A and B: Rate = k[A][B].
- Termolecular elementary reaction (A + B + C → products): The rate law is proportional to the product of the concentrations of reactants A, B, and C: Rate = k[A][B][C]. Termolecular reactions are relatively rare due to the low probability of three molecules simultaneously colliding with the correct orientation and energy.
Deriving Rate Laws: A Step-by-Step Approach
The process of deriving the rate law from a mechanism involves several crucial steps:
-
Identify the Elementary Steps: Carefully examine the proposed mechanism and break down the overall reaction into its constituent elementary steps. This includes identifying intermediates and catalysts, if present.
-
Write the Rate Law for Each Elementary Step: Using the molecularity of each elementary step, write its corresponding rate law. Remember, for an elementary reaction, the rate law is directly proportional to the product of the reactant concentrations raised to their stoichiometric coefficients.
-
Identify the Rate-Determining Step (RDS): This is the slowest step in the mechanism. The overall rate law is typically determined by the rate law of the RDS. However, this isn't always the case; sometimes, the overall rate law depends on the concentrations of pre-equilibrium steps.
-
Express Intermediate Concentrations in Terms of Reactants: The rate law should only include concentrations of reactants that appear in the overall balanced equation. If the rate law for the RDS contains intermediates, you need to express their concentrations in terms of reactants using the equilibrium expressions from faster steps (pre-equilibrium assumption).
-
Combine and Simplify: Substitute the expressions obtained in step 4 into the rate law for the RDS and simplify the resulting equation. This will give you the overall rate law for the reaction.
Examples: Illustrating the Process
Let's illustrate this process with some examples:
Example 1: Simple Consecutive Reactions
Consider the following mechanism for the decomposition of ozone:
Step 1: O₃ → O₂ + O (slow) Step 2: O₃ + O → 2O₂ (fast)
In this case, Step 1 is the rate-determining step. The rate law is simply:
Rate = k₁[O₃]
Since there are no intermediates appearing in the rate law for the RDS, we don't need to use equilibrium approximations. The overall rate law is first order with respect to ozone.
Example 2: Mechanism with an Intermediate
Consider the reaction: A + B → C, with the following mechanism:
Step 1: A + B ⇌ AB* (fast equilibrium) Step 2: AB* → C (slow)
Here, AB* is an intermediate. Step 2 is the RDS. The rate law for Step 2 is:
Rate = k₂[AB*]
Since AB* is an intermediate, we need to express its concentration in terms of reactants A and B. From the fast equilibrium in Step 1, we have:
K = [AB*]/[A][B] where K is the equilibrium constant for Step 1.
Therefore, [AB*] = K[A][B]. Substituting this into the rate law for Step 2:
Rate = k₂K[A][B] = k[A][B]
where k = k₂K. The overall rate law is second-order, first-order with respect to both A and B.
Example 3: A More Complex Case
Let's analyze a more complex mechanism involving multiple steps and pre-equilibrium:
Step 1: A + B ⇌ AB (fast equilibrium, K1) Step 2: AB + C → D (slow, k2) Step 3: D → E (fast)
The rate-determining step is step 2. The rate law for this step is:
Rate = k2[AB][C]
AB is an intermediate. We use the equilibrium expression from step 1:
K1 = [AB]/[A][B] => [AB] = K1[A][B]
Substituting this into the rate law for step 2 gives us:
Rate = k2*K1[A][B][C] = k[A][B][C]
where k = k2*K1. The overall reaction rate is first-order with respect to A, B, and C.
Steady-State Approximation
For more complex mechanisms, the pre-equilibrium assumption might not be valid. In such cases, the steady-state approximation is used. This approximation assumes that the concentration of any intermediate remains relatively constant during the reaction. This means that the rate of formation of the intermediate equals its rate of consumption. This allows us to express the intermediate concentration in terms of reactant concentrations and then substitute it into the rate law for the RDS.
Common Mistakes and Pitfalls
-
Confusing Overall Reaction with Elementary Steps: The overall stoichiometry does not directly dictate the rate law. The rate law is determined by the mechanism.
-
Incorrectly Identifying the RDS: Identifying the RDS is crucial. If you choose the wrong step, your derived rate law will be incorrect.
-
Ignoring Intermediates: Intermediates must be expressed in terms of reactants using appropriate equilibrium or steady-state approximations.
-
Incorrect Application of Equilibrium and Steady-State Approximations: These approximations have specific requirements and must be applied correctly.
Conclusion: Mastering the Art of Rate Law Derivation
Deriving the rate law from a proposed mechanism is a fundamental skill in chemical kinetics. By systematically following the steps outlined above and practicing with various examples of increasing complexity, you can develop a strong understanding of how to connect microscopic events to macroscopic observable rates. Remember that understanding the assumptions of pre-equilibrium and steady-state approximations is vital for tackling more complex mechanisms accurately. Mastering this skill opens the door to further exploration of reaction dynamics and the design of efficient catalytic processes. The ability to confidently predict and analyze reaction rates is invaluable in numerous chemical applications, from industrial catalysis to environmental chemistry. Through diligent study and practice, you can become proficient in this crucial area of chemistry.
Latest Posts
Latest Posts
-
Reinforcement For Emitting A Correct Echoic Is Usually
Mar 29, 2025
-
C Is Trying To Determine Whether To Convert
Mar 29, 2025
-
A Female Infant Is In For A Feeding Consultant
Mar 29, 2025
-
Nutrient Cycling In The Serengeti Answer Key
Mar 29, 2025
-
The Client Record Houses The Following Information Except
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Writing The Rate Law Implied By A Simple Mechanism . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
