Protons Neutrons And Electrons Practice Worksheet Answer Key
Onlines
Mar 26, 2025 · 5 min read
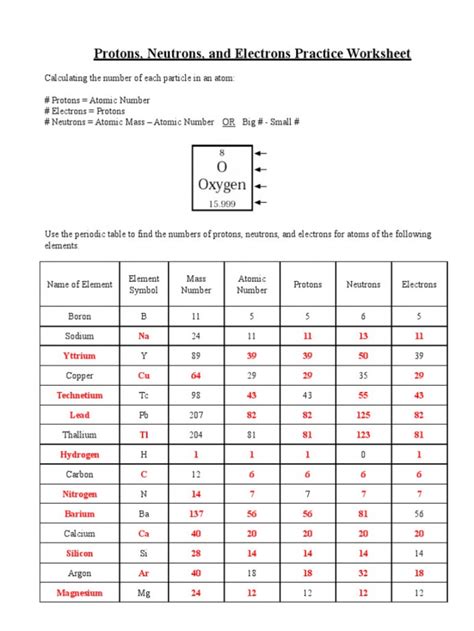
Table of Contents
Protons, Neutrons, and Electrons Practice Worksheet: A Comprehensive Guide with Answer Key
Understanding the fundamental building blocks of matter – protons, neutrons, and electrons – is crucial for grasping the basics of chemistry and physics. This comprehensive guide serves as a practice worksheet answer key, providing detailed explanations and solutions to common problems encountered when working with atomic structure. We'll explore the properties of each subatomic particle, delve into atomic number and mass number calculations, and address isotopic variations.
Understanding Subatomic Particles: Protons, Neutrons, and Electrons
Atoms, the basic units of matter, are composed of three primary subatomic particles:
1. Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's atomic number and dictates its chemical properties. The proton's charge is +1.
2. Neutrons: Neutral particles (no charge) also located in the atom's nucleus. They contribute to the atom's mass but not its charge.
3. Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom. The electron's charge is -1.
Key Differences Summarized:
| Particle | Charge | Location | Mass (amu) (approx.) |
|---|---|---|---|
| Proton | +1 | Nucleus | 1 |
| Neutron | 0 | Nucleus | 1 |
| Electron | -1 | Electron Shells | ~0 (negligible) |
Atomic Number and Mass Number: Calculations and Interpretations
Atomic Number (Z): This represents the number of protons in an atom's nucleus. It uniquely identifies an element on the periodic table. For example, hydrogen (H) has an atomic number of 1, meaning it has one proton.
Mass Number (A): This is the total number of protons and neutrons in an atom's nucleus. It represents the atom's approximate mass. To calculate the mass number, simply add the number of protons and neutrons: A = protons + neutrons.
Isotopes: Variations in Neutron Count
Isotopes are atoms of the same element (same number of protons) that have different numbers of neutrons. This means they have the same atomic number but different mass numbers. For example, carbon-12 (¹²C) has 6 protons and 6 neutrons, while carbon-14 (¹⁴C) has 6 protons and 8 neutrons. Both are isotopes of carbon.
Practice Problems and Solutions
Let's work through some practice problems to solidify your understanding of protons, neutrons, and electrons.
Problem 1: An atom of oxygen has 8 protons and 8 neutrons. What is its atomic number, mass number, and number of electrons?
Solution:
- Atomic Number (Z): 8 (equal to the number of protons)
- Mass Number (A): 16 (8 protons + 8 neutrons)
- Number of Electrons: 8 (equal to the number of protons in a neutral atom)
Problem 2: An atom has an atomic number of 11 and a mass number of 23. How many protons, neutrons, and electrons does it have?
Solution:
- Protons: 11 (equal to the atomic number)
- Neutrons: 12 (mass number - atomic number = 23 - 11)
- Electrons: 11 (equal to the number of protons in a neutral atom)
Problem 3: Two isotopes of chlorine exist: chlorine-35 and chlorine-37. Chlorine has an atomic number of 17. How many protons and neutrons are present in each isotope?
Solution:
- Chlorine-35:
- Protons: 17 (atomic number)
- Neutrons: 18 (mass number - atomic number = 35 - 17)
- Chlorine-37:
- Protons: 17 (atomic number)
- Neutrons: 20 (mass number - atomic number = 37 - 17)
Problem 4: Identify the element with 19 protons.
Solution: Potassium (K). You would consult the periodic table to find the element with atomic number 19.
Problem 5: A neutral atom has 20 electrons. What is its atomic number?
Solution: 20. In a neutral atom, the number of electrons equals the number of protons, which is the atomic number.
Problem 6: An ion has 17 protons, 18 neutrons, and 18 electrons. What is its charge?
Solution: -1. The ion has one more electron than protons (18 - 17 = 1), resulting in a negative charge.
Problem 7: Calculate the number of neutrons in Uranium-238, given that its atomic number is 92.
Solution: 146. (Mass number - atomic number = 238 - 92)
Problem 8: Explain the difference between atomic number and mass number. Provide an example.
Solution: The atomic number is the number of protons in an atom's nucleus, defining the element. The mass number is the total number of protons and neutrons in the nucleus. For example, Carbon-12 has an atomic number of 6 (6 protons) and a mass number of 12 (6 protons + 6 neutrons).
Problem 9: What are isotopes, and how do they differ from each other? Give an example.
Solution: Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. This results in different mass numbers. For example, Carbon-12 and Carbon-14 are isotopes of carbon. Both have 6 protons, but Carbon-12 has 6 neutrons, and Carbon-14 has 8 neutrons.
Problem 10: Why is the mass of an electron considered negligible compared to the mass of a proton or neutron?
Solution: The mass of an electron is approximately 1/1836 the mass of a proton or neutron. This difference is so significant that the electron's mass is often disregarded when calculating the mass number of an atom.
Advanced Concepts and Further Practice
This section briefly introduces more advanced concepts related to atomic structure and provides avenues for further practice.
1. Electron Configuration: Electrons occupy specific energy levels or shells around the nucleus. Understanding electron configuration helps predict an element's chemical behavior.
2. Ions: Atoms that have gained or lost electrons, resulting in a net positive (cation) or negative (anion) charge.
3. Nuclear Chemistry: Deals with the nucleus and its transformations, including radioactivity and nuclear reactions.
4. The Periodic Table: This organized chart of elements provides valuable information about atomic structure and properties.
To further enhance your understanding, you can:
- Consult textbooks: Numerous chemistry and physics textbooks offer comprehensive coverage of atomic structure.
- Utilize online resources: Many educational websites and online simulations provide interactive exercises and tutorials.
- Work through additional practice problems: Find more practice worksheets online or in textbooks.
By consistently practicing and reviewing these concepts, you'll develop a strong foundation in understanding protons, neutrons, and electrons—the fundamental building blocks of matter. Remember to utilize the periodic table as a key resource for finding atomic numbers and other element-specific information. Consistent practice is key to mastering this essential aspect of chemistry and physics.
Latest Posts
Latest Posts
-
3 3 7 Connect Patch Panel Cables 2
Mar 26, 2025
-
Le Pidieron Los Menus Al Camarero
Mar 26, 2025
-
Rn Adult Medical Surgical Myocardial Infarction Complications
Mar 26, 2025
-
Chapter 1 History And Trends Of Healthcare
Mar 26, 2025
-
Summary Of Chapter 5 The Giver
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Protons Neutrons And Electrons Practice Worksheet Answer Key . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
