When Does The Net Flux Of Dissolved Molecules Stop
Onlines
Mar 30, 2025 · 5 min read
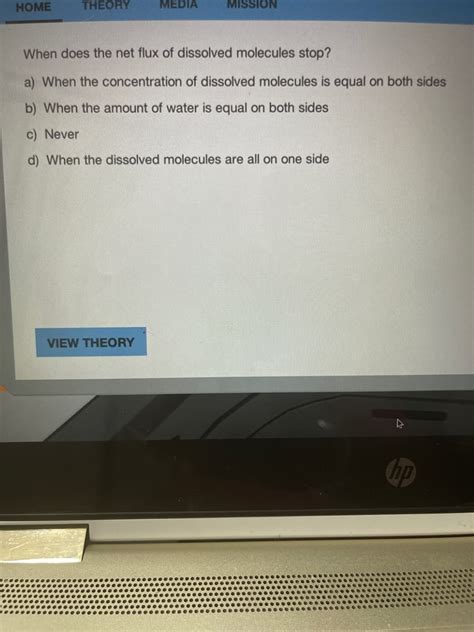
Table of Contents
When Does the Net Flux of Dissolved Molecules Stop? Understanding Equilibrium and Diffusion
The seemingly simple question, "When does the net flux of dissolved molecules stop?" delves into the fundamental principles of physical chemistry and biology. Understanding this requires grasping the concepts of diffusion, concentration gradients, equilibrium, and the factors that influence molecular movement. This article will explore these concepts in detail, providing a comprehensive explanation of when and why the net movement of dissolved molecules ceases.
Diffusion: The Driving Force Behind Molecular Movement
Diffusion is the spontaneous net movement of particles from a region of higher concentration to a region of lower concentration. This movement is driven by the inherent kinetic energy of molecules—they are constantly in motion, colliding with each other and their surroundings. This random motion, in the absence of other forces, leads to a gradual equalization of concentration. Imagine dropping a drop of ink into a glass of water; initially, the ink is highly concentrated in one area. Over time, however, the ink molecules spread out, eventually distributing themselves evenly throughout the water. This is diffusion in action.
Factors Affecting Diffusion Rate
Several factors influence the rate at which diffusion occurs:
-
Concentration Gradient: The steeper the concentration gradient (the larger the difference in concentration between two regions), the faster the rate of diffusion. A large difference in concentration provides a stronger driving force for molecular movement.
-
Temperature: Higher temperatures increase the kinetic energy of molecules, leading to faster diffusion. Molecules move more rapidly at higher temperatures, increasing the frequency of collisions and the rate of spread.
-
Molecular Size and Shape: Smaller molecules generally diffuse faster than larger ones because they can navigate through the spaces between solvent molecules more easily. Similarly, the shape of a molecule can affect its ability to move through a medium.
-
Solvent Viscosity: The viscosity (thickness) of the solvent also plays a role. Diffusion is slower in more viscous solvents because the molecules encounter more resistance as they move.
-
Membrane Permeability (in biological systems): In biological systems, the permeability of cell membranes significantly impacts the rate of diffusion. Only molecules that can cross the membrane can diffuse across it.
Reaching Equilibrium: The Cessation of Net Flux
The net flux of dissolved molecules stops when equilibrium is reached. Equilibrium is a state where the concentration of the dissolved molecules is uniform throughout the system. At equilibrium, there is still molecular movement—molecules continue to move randomly—but there is no longer a net movement from one region to another. The rate of movement from a high-concentration region to a low-concentration region is equal to the rate of movement in the opposite direction.
It's crucial to understand the distinction between net flux and individual molecular movement. Even at equilibrium, individual molecules are still moving, but their movement is random and balanced, resulting in no overall change in concentration.
Think of it like a busy highway. Even when the traffic flow is balanced (cars moving equally in both directions), individual cars are still in motion. But there's no net movement of cars in any particular direction. Equilibrium is a dynamic state, not a static one.
Beyond Simple Diffusion: Other Factors Influencing Molecular Movement
While simple diffusion based on concentration gradients is a primary driver of molecular movement, several other factors can influence the net flux and the time it takes to reach equilibrium:
-
Active Transport: Biological systems often utilize active transport mechanisms to move molecules against their concentration gradients. This process requires energy (typically ATP) and can override the effects of simple diffusion. Active transport can maintain concentration differences across membranes even when equilibrium would otherwise be expected.
-
Osmosis: Osmosis is the movement of water molecules across a semipermeable membrane from a region of higher water concentration (lower solute concentration) to a region of lower water concentration (higher solute concentration). Osmosis plays a significant role in maintaining fluid balance in biological systems. It's a specific type of diffusion involving water.
-
Facilitated Diffusion: In facilitated diffusion, membrane proteins help transport molecules across cell membranes, often along the concentration gradient. This process speeds up the rate of diffusion for certain molecules that cannot readily cross the membrane on their own. However, the net flux still stops when equilibrium is achieved.
-
Electrochemical Gradients: In the case of charged molecules (ions), the electrochemical gradient – the combined influence of the concentration gradient and the electrical potential difference – determines the net movement of these molecules. This is particularly important in nerve cells and other electrically excitable tissues.
-
Pressure Gradients: Pressure differences can also drive molecular movement. For example, filtration across a membrane driven by pressure is not a diffusion process but results in a net movement of molecules that could influence equilibrium conditions.
Practical Implications and Applications
Understanding when net flux stops has significant implications across various scientific fields:
-
Pharmacology: Drug delivery relies heavily on the principles of diffusion and equilibrium. The rate at which a drug reaches its target site depends on its ability to diffuse across membranes and the concentration gradient.
-
Environmental Science: Understanding the diffusion of pollutants in water bodies or the atmosphere is crucial for environmental monitoring and remediation efforts.
-
Food Science: The preservation of food often involves controlling the diffusion of water and other molecules to prevent spoilage.
-
Materials Science: The diffusion of atoms in solids is fundamental to many material properties, including strength and conductivity.
-
Cell Biology: The ability of cells to maintain internal environments different from their surroundings relies heavily on regulated diffusion and other transport mechanisms. This is crucial for cell function and survival.
Conclusion: A Dynamic Equilibrium
The net flux of dissolved molecules stops when equilibrium is reached. This is a dynamic state where the concentration is uniform, and there is no net movement of molecules from one region to another. However, individual molecules continue their random motion. Reaching equilibrium is not simply about cessation of movement, it's about balance of movement, and numerous factors such as temperature, concentration gradients, membrane permeability, active transport, and other forms of transport can influence the time it takes to reach this state and the final equilibrium concentrations. Understanding this dynamic balance is essential in a variety of scientific disciplines and has numerous applications in everyday life.
Latest Posts
Latest Posts
-
How Do Financial Capital Markets Transform Financial Capital Flows
Apr 01, 2025
-
Which Of The Following Statements About The Amygdala Is Correct
Apr 01, 2025
-
Consider A Binomial Experiment With N 20 And P 0 70
Apr 01, 2025
-
What Big Problem Does Power Query Solve
Apr 01, 2025
-
What Are The Origins Of Appearance Enhancement
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about When Does The Net Flux Of Dissolved Molecules Stop . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
